Abstract
Despite acting as a barrier for the organs they encase, epithelial cells turn over at some of the fastest rates in the body. However, epithelial cell division must be tightly linked to cell death to preserve barrier function and prevent tumour formation. How does the number of dying cells match those dividing to maintain constant numbers? When epithelial cells become too crowded, they activate the stretch-activated channel Piezo1 to trigger extrusion of cells that later die1. However, it is unclear how epithelial cell division is controlled to balance cell death at the steady state. Here we show that mammalian epithelial cell division occurs in regions of low cell density where cells are stretched. By experimentally stretching epithelia, we find that mechanical stretch itself rapidly stimulates cell division through activation of the Piezo1 channel. To stimulate cell division, stretch triggers cells that are paused in early G2 phase to activate calcium-dependent phosphorylation of ERK1/2, thereby activating the cyclin B transcription that is necessary to drive cells into mitosis. Although both epithelial cell division and cell extrusion require Piezo1 at the steady state, the type of mechanical force controls the outcome: stretch induces cell division, whereas crowding induces extrusion. How Piezo1-dependent calcium transients activate two opposing processes may depend on where and how Piezo1 is activated, as it accumulates in different subcellular sites with increasing cell density. In sparse epithelial regions in which cells divide, Piezo1 localizes to the plasma membrane and cytoplasm, whereas in dense regions in which cells extrude, it forms large cytoplasmic aggregates. Because Piezo1 senses both mechanical crowding and stretch, it may act as a homeostatic sensor to control epithelial cell numbers, triggering extrusion and apoptosis in crowded regions and cell division in sparse regions.
Similar content being viewed by others
Main
To investigate how epithelial cell division is controlled at steady state, we seeded Madin–Darby canine kidney (MDCK) epithelial cells and measured the percentage of mitotic cells daily by immunostaining cells for phospho-histone H3 (H3P) (Extended Data Fig. 1a, b). Although epithelial cells never stop dividing, the rates of cell division reach a slow steady state by around day 5, at an average density of 11 cells per 1,000 μm2, 3 days after reaching confluence (Extended Data Fig. 1a, asterisk). The approximately 7% mitotic rate at seeding slows to 0.7% at steady state, when most cells are in the G0/G1 stage of the cell cycle (Extended Data Fig. 1a–c and Supplementary Videos 1, 2).
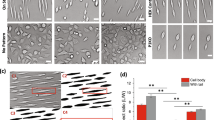
How epithelial cells regulate cell division once they reach an optimal density is unclear. Although the overall cell division rates decrease as the monolayer reaches steady state, videos reveal that cells divide in sparse sub-regions of the epithelium (Supplementary Video 2). Furthermore, cell division occurs in zones that are consistently around 1.6-fold less dense than zones in which no division occurs, as quantified by cell lengths in dividing versus non-dividing regions in human colon crypts (1.6-fold), zebrafish epidermis (1.5-fold), and MDCK monolayers (1.7-fold) (Supplementary Videos 2, 3 and Extended Data Fig. 2). These observations led us to wonder whether cell stretch due to low cell density could activate epithelial cell division. To test this hypothesis, we experimentally stretched MDCK cells at the steady state by either wounding or directly stretching cells in plates designed for uniaxial tension, and analysed mitotic rates at different times after stretch. Stretching cells 1.4-fold using a previously published device1 or a newly designed stretch device (Extended Data Fig. 3a) was sufficient to induce an approximately 5-fold increase in cell division within only 1 h (Extended Data Fig. 3b and Fig. 1a). Although the increased proliferation rate was low (1.3%), it returned cells to homeostatic densities, as measured by averaged cell lengths, within 4 h (Fig. 1b). Furthermore, scratching an MDCK monolayer caused cells to stretch 2.5-fold times their original length once cell migration ceased and triggered a wave of cell division (Fig. 1c, d and Supplementary Video 4, n = 6), similar to that seen previously2. Cell division typically occurred within 1–2 h of wound closure, similar to the kinetics occurring after stretch.
a, b, Proliferation rates (a) and cell lengths (b) at various times after stretch show that stretch-induced cell divisions return cell densities to control (ctrl) levels. Values are means of 6 areas each from 3 experiments, error bars denote s.e.m. *P < 0.01, **P < 0.005, ***P < 0.0005, unpaired t-tests compared to control. c, Phase-contrast images showing cumulatively where and when cells divide (red dots) during wound healing of an MDCK monolayer, with time in hours. Wound edge is highlighted by the white line. d, Cell divisions after monolayer wounding. Arrow denotes wound closure. Graph in d is representative of 14 experiments.
To determine what regulates stretch-induced mitosis, we inhibited a variety of candidate proteins implicated in cellular stress or stretch responses. Gadolinium, a generic stretch-activated channel inhibitor, consistently blocked stretch-induced proliferation, compared to a generic inhibitor of mitosis, the CDK1 inhibitor roscovitine (Fig. 2a). Because Piezo1 is a stretch-activated channel required for crowding-induced, extrusion-dependent epithelial cell death1,3, we tested whether it might also control stretch-dependent proliferation. Knockdown of Piezo1 by short interfering RNA (siRNA; or a short hairpin RNA (shRNA) Piezo1 construct, not shown) reduced Piezo1 to 7% of wild-type levels and prevented stretch-induced mitosis (Fig. 2a, b). Notably, long-term knockdown of Piezo1 also reduced steady-state rates of proliferation (Fig. 2a, unstretched). Transfection of a green fluorescent protein (GFP)-tagged Piezo1 construct not targeted by the siRNA rescued stretch-induced proliferation, indicating that proliferation after stretch requires Piezo1 (Fig. 2a, b). Piezo1 knockdown also blocked cell division after wound closure, in comparison to control monolayers (Fig. 2c). To test whether Piezo1 controlled epithelial cell division in vivo, we examined its role in zebrafish epidermis, a model for the simple epithelia that coat our organs. We assayed for H3P-positive cells in epidermis from 4-day-old zebrafish larvae, a stage at which cell division reaches a steady-state rate (Extended Data Fig. 4a and ref. 1). Using a CRISPR-based technique to knockdown Piezo1 mosaically in the F0 generation4,5, we found that reducing Piezo1 levels to approximately 50% decreased the number of H3P-positive epidermal cells by 5-fold (Fig. 2d–f). Furthermore, a photo-activated morpholino targeting Piezo1 also reduced epidermal cell proliferation by around 5-fold (Extended Data Fig. 4b–d). Together, our data show that epithelial cells at the steady state become stretched before they divide, that stretch is sufficient to activate cell division rapidly, and that cell division either at the steady state or after experimental stretch requires the stretch-activated channel Piezo1.
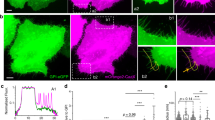
a, Inhibition or siRNA-mediated knockdown (KD) of Piezo1 blocks stretch-induced mitosis, and is rescued by GFP–Piezo1. Values are means of 6 measurements each from 5 experiments. Gd3+, gadolinium; rosco., roscovitine; scr. siRNA ctrl, scrambled siRNA control. b, Immunoblot confirming knockdown and rescue (Supplementary Fig. 1 for full blots). c, Piezo1 knockdown blocks cell division after wound closure. Graphs are representative of 20 similar experiments. d–f, CRISPR-mediated Piezo1 knockdown reduces Piezo1 mRNA by 50% (n = 3) (d) and markedly decreases mitosis rates (e, f), measured in 26 wild-type and 53 knockout 4-day-old zebrafish larvae. Scale bar, 100 μm. Images are representative of a total of 12 images for each condition. Error bars in a, d and e denote s.e.m. **P < 0.005, ***P < 0.0005, ****P < 0.0001, unpaired t-test.
We next tested where in the cell cycle stretch activates Piezo1 to induce mitosis. Because the mitotic response to stretch is so rapid, it seemed unlikely that stretch-activation of Piezo1 triggers cells to transit through an entire cell cycle from the G0 phase (~12–24 h). Indeed, 2 h of stretch did not increase the number of cells entering S phase, as measured by EdU incorporation (Extended Data Fig. 5a). Moreover, other reports find that stretch induces epithelial cells to enter the cell cycle but reach G1 after only 6 h6,7,8. To determine whether stretch activates mitosis at G2 or M, we tested whether stretch stimulates cyclin B, which accumulates in the cytoplasm at G2. To prevent cyclin B degradation as cells shift into mitosis, we measured cytoplasmic cyclin B accumulation in the presence of the CDK1 inhibitor, roscovitine. We found that stretch induces cytoplasmic cyclin B accumulation over time (Fig. 3a and Extended Data Fig. 5b). Inhibiting Piezo1 with gadolinium or transcription with α-amanitin blocks stretch-induced cyclin B synthesis (Fig. 3a), suggesting that stretch-induced cyclin B induction requires both Piezo1 and transcription. However, because Piezo1 is a transmembrane protein that relays calcium in response to mechanical stress3,9,10,11, it probably activates transcription indirectly. Inhibiting calcium with either ruthenium red or BAPTA-AM blocked stretch-induced mitosis and cyclin B production, respectively, confirming that Piezo1 acts through calcium to activate mitosis (Fig. 3b, c). Videos of MDCK monolayers expressing CMV-R-GECO1 (ref. 12) reveal that a single spark of calcium precedes mitosis entry (noted by physiological cell rounding) by 65 min (Extended Data Fig. 5b, c). Because both cyclin B and mitosis require Piezo1 and calcium influx (Fig. 3b, c), we next investigated what induces proliferation in response to calcium influx. A top candidate is the extracellular signal-regulated kinase (ERK1), a calcium-activated target of MEK1/2 (refs 13, 14, 15) with roles in controlling G2/M16,17,18. We found that stretch-induced calcium activates ERK1/2 (measured by phospho-ERK1/2 immunoblot) within only 5 min, but is blocked by gadolinium addition (Fig. 3d). Using the MEK1/2 inhibitor AZD6244 (and U0126, not shown), we found that both stretch-induced mitosis and synthesis of cyclin B require the ERK1/2–MEK1/2 pathway (Fig. 3b, c). Thus, we propose that stretch causes Piezo1 to trigger calcium currents that activate ERK1/2 via MEK1/2, which triggers cyclin B transcription and initiate mitosis (Fig. 3e).
a, Stretch induces cyclin B accumulation (with CDK1 inhibition), which is blocked by Gd3+ or α-amanitin. Values are means from 6 experiments, error bars denote s.e.m. P < 0.0001, two-way analysis of variance (ANOVA). b, c, Inhibiting intracellular calcium (using BAPTA-AM or ruthenium red) or inhibiting MEK1/2 (using AZD6244) blocks stretch-induced mitosis (b) and cytoplasmic cyclin B (with CDK1 inhibition) (c). Data are means of 6 areas each from 3 experiments, error bars denote s.e.m. ***P < 0.002, ****P < 0.0001, unpaired t-test. NS, non-stretched; S, stretched. d, Stretch rapidly activates ERK1/2 phosphorylation (p-ERK1/2) in a Piezo1-dependent manner (see Supplementary Fig. 1 for full scans). Time is shown in minutes. e, Model: stretch triggers cells in early G2 phase to activate Piezo1-dependent calcium influx, which stimulates ERK1/2-dependent transcription of cyclin B and cell division.
It is curious that one protein, Piezo1, can control two opposing processes—cell division and cell death—to regulate cell numbers. This seems especially surprising, given that Piezo1 triggers opposing outcomes through the same second messenger: calcium. However, the type of mechanical tension is crucial for the type of response: we find that stretch induces cell division but not cell extrusion, whereas crowding induces cell extrusion but not cell division (Fig. 4a, b). Notably, the magnitude of mechanical strain for each response is similar: 1.5–1.7-fold stretch induces cell division whereas 1.7–1.8-fold crowding induces cell extrusion1 (Extended Data Fig. 6a). Similar paradoxical signalling controls opposing outcomes in other systems, but typically through secreted molecules19,20,21. For instance, IL-2 maintains homeostatic T cell numbers by controlling both cell division and cell death depending on the levels secreted21. Thus, Piezo1 signalling through calcium may represent a new mechanism for regulating a paradoxical feedback loop. While the benefit of having a single regulator control two opposing processes is that its loss should downregulate both processes, Piezo1 mutations and misexpression frequently found in colorectal, gastric and thyroid cancers suggest that Piezo1 may act as a tumour suppressor22,23,24,25. Future work is needed to determine whether Piezo1 misregulation is sufficient to drive tumorigenesis, and if the relevant mutations affect both epithelial cell death and division.
a, b, Stretch triggers only mitosis (a), whereas crowding induces only cell extrusion/death (b). Data are means of 6 fields of view each from 4 experiments, error bars denote s.e.m. c, d, Experimental induction of calcium influx induces proliferation (n = 8; c) but not extrusion/death (n = 9; d) within 2 h. ****P < 0.0001, unpaired t-test. e, Piezo1 (green) shifts from the nuclear envelope in cells at low density to the cytoplasm/plasma membrane in cells at high density, forming cytoplasmic aggregates. f, Piezo1 disappears after stretch or wounding, eventually localizing to the nuclear envelope (arrows, wound). The images are representative of 10 images for each condition. Scale bars, 10 μm.
We next sought to determine how Piezo1 differentially interprets crowding versus stretching. Cellular responses could depend on how the cell is primed, the levels and localization of Piezo1, as well as the type of tension. Our findings show that stretch-activation of Piezo1 target cells primed in early G2 to divide rapidly. It is not clear whether Piezo1 also collaborates with the transcriptional regulators Yap, Taz and β-catenin upon stretch to promote cell cycle entry at the G1 phase6,8, although it is a tempting prospect, since tension-dependent nuclear translocation of Yap requires Piezo1 in neuronal stem cells26. Older, crowded cells prone to extrusion may be post-mitotic, as typically seen in villus tips, and less likely to divide in response to calcium. The type of mechanical tension may also have a role in how Piezo1 signals. We find that while chemical activation of calcium currents is sufficient to activate cell division, it is not sufficient to promote cell extrusion and death (Fig. 4c, d). Thus, either thapsigargin or the calcium ionophore A23187 activates calcium at sites required for mitosis, or crowding-induced extrusion requires mechanical crowding in addition to calcium currents. Finally, the ability of Piezo1 to sense different types of tension may depend on its subcellular localization and levels. At subconfluence, Piezo1 levels are low, mainly localizing to the nuclear envelope, but accumulate over time. In confluent but sparser epithelial regions prone to divide, Piezo1 localizes to the plasma membrane and endoplasmic reticulum, whereas in crowded regions prone to extrude, Piezo1 develops into large cytoplasmic aggregates (Fig. 4e). Notably, although Piezo1 accumulates over time, stretching dense MDCK monolayers either mechanically or by wounding causes Piezo1 levels to drop markedly and eventually relocalize to the nucleus (Fig. 4f). At lower densities, when Piezo1 is predominantly at the nucleus, stretch does not appear to have a significant role in regulating mitosis (Extended Data Fig. 7b, c), potentially because the division rate is already very high. In this way, Piezo1 levels and localization could adjust to regulate cell numbers once cells reach a steady state: in sites where cells are sparse, Piezo1 localizes to sites where it may sense stretch, whereas in sites where cells are older and crowded, Piezo1 accumulates into structures where it may sense crowding (Extended Data Fig. 6b). Excessive cell death or wounding may reset the cell clock by degrading Piezo1 so that it would start measuring cell numbers only once cells reach the steady state again. Future studies will need to test whether Piezo1 abundance and localization control its response to different mechanical forces.
Our work has defined a role for mechanical tensions regulating constant epithelial cell numbers, essential for maintaining a tight barrier and preventing cancers from forming. We have found that Piezo1 acts to link the number of cells dying with those dividing by measuring tensions. If epithelial cells become too dense, crowding induces some cells to extrude and die, but if they become too sparse, stretch triggers some cells to divide rapidly to equilibrate epithelia to optimal densities.
Methods
Cell culture and stretching assays
MDCK II cells (tested for mycoplasma but not authenticated) (gift from K. Matlin) were cultured in DMEM high glucose with 5% FBS (Atlas, Biologicals) and 100 μg ml−1 penicillin/streptomycin (Invitrogen) at 5% CO2, 37 °C. Cell stretching was done using a custom-designed Teflon chamber fabricated to culture cells on a flexible silicone membrane (2 × 2.4 cm, 0.5 mm thickness)1. Before cell seeding, 2 × 2.4 cm silicone membranes were stretched and coated with 5 μg ml−1 fibronectin (Sigma) at 37 °C for 1 h. To stretch membranes with our device, one edge of the silicone membrane was clamped in place, while the other side was clamped to a movable shaft, which moves through a Teflon chamber with a sealing gasket. The movable shaft was pulled out between 2 and 6 mm, resulting in stretch of 10–33%. Approximately 2.5 × 106 cells were plated onto the 2 × 2.4 cm silicone matrices in a neutral taut state in the device and grown to confluence, then stretched to desired lengths with control chambers being left in a neutral state. Epithelial monolayers were fixed in stretched or neutral state and stained. As an alternative stretching method, 2 × 106 cells were grown to confluence in pronectin-coated flexcell plates designed for uniaxial stretch (Flexcell International Corporation), and stretched by clamping onto a custom-designed base plate (publicly available at https://www.shapeways.com/ as ‘Narrow Bump Base in white strong flexible nylon plastic’) using two ‘Clamp Bars in Stainless Steel’ (Shapeways). The clamp bars and base must be tapped with a 0.25 inch (6.35 mm)-20 tap to match steel 0.25 inch-20 fully threaded studs and rods and capped with knurled style thumbscrews (both Essentra Components).
Wounding assays and live cell imaging
Control MDCK II cells or those expressing a pTRIPZ piezo1 shRNA construct were grown to confluence in 24-well glass bottom dishes (MatTek) or 8-chamber slides (Labtek, Fisher Scientific). Cells were treated with 1.5 μg ml−1 doxycycline (Sigma) for 48 h to knockdown Piezo1. For wounding experiments, monolayers were scratched with a 10-μl pipet tip. Cells were imaged in complete DMEM supplemented with 10 mM HEPES (Invitrogen) on a Nikon TE200 inverted microscope at 37 °C or an EVOS FL Auto with a Stable Z controller heated stage (Bioptechs) at 37 °C. Time-lapse videos were analysed using ImageJ.
For calcium imaging, MDCK cells were transfected with CMV-R-GECO1, a gift from R. Campbell (Addgene plasmid 32444)12 and imaged continuously (3=s intervals) for 4 h. For cell cycle imaging, MDCK cells were transfected with pEGFP-PCNA-IRES-puro2b, a gift from D. Gerlich (Addgene plasmid 26461)1. Cells were imaged with a Nikon wide-field microscope at 37 °C coupled to an Andor Clara interline CCD camera.
Drug treatments
The following drugs were used in experiments: 10 μM gadolinium (iii) chloride (for both zebrafish and cell culture) to block Piezo1, and 30 μM ruthenium red or 10 μM BAPTA-AM was used to inhibit calcium (all from Sigma); 10 μM roscovitine was used to block CDK1; 5 μM α-amanitin (Sigma) was used to inhibit RNA polymerase II and III; 1 μM AZD6244 (Cayman Chemical) or 1 μM U0126 (Sigma) was used to inhibit MEK1 and MEK2; 1 μM A23187 or 5 μM thapsigargin (both from Sigma) was used to induce calcium influx.
Cell immunostaining
Cells were fixed with 4% formaldehyde in PBS at room temperature for 20 min. Fixed cells were rinsed three times in PBS, permeabilized for 10 min with PBS containing 0.5% Triton X-100, and blocked in AbDil (PBS with 0.1% Triton X-100 and 2% BSA), then incubated in primary antibody (in AbDil) for 1 h, washed three times with PBS and 0.1% Triton X-100, and incubated in secondary antibodies. Antibody concentrations used for immunostaining were: 1:1,000 mouse phospho-histone H3 (S10) (Cell Signaling Technology); 1:1,000 rabbit histone H3 (phospho S10) (Abcam); 1:200 mouse β-catenin (Cell Signaling Technology); 1:200 rabbit cyclin B1 XP (Cell Signaling Technology); 1:200 rabbit Piezo1 (Novus, NBP1-78446). Only this Piezo1 antibody reliably worked for Piezo1 immunostaining, as it vanishes in cells mosaically expressing a Piezo1-shRNA-mCherry construct (Extended Data Fig. 5a). Actin was detected using 0.1 μg ml−1 Alexa Fluor 647–phalloidin (Invitrogen). Replicating DNA was detected using a Click-it EdU labelling kit, according to the manufacturer’s protocol (Life Technologies). Alexa Fluor 488, 568 and 647 goat anti-mouse and anti-rabbit IgG were used as secondary antibodies (Invitrogen). DNA was detected with 1 μg ml−1 DAPI in all fixed cell experiments.
Fluorescent microscopy
Images were captured on a Nikon Eclipse 90i using a ×40 plan fluor 0.75 lens with a Retiga 2000R cooled mono 12-bit camera (Q Imaging) driven by NIS Elements (Nikon) or on a Nikon Eclipse TE300 inverted microscope converted for spinning disc confocal microscopy (Andor Technologies) using a 20× or 60× plan fluor 0.95 oil lens with an electron-multiplied cooled CCD camera 1,000 × 1,000, 8 × 8 mm2 driven by the IQ software (Andor Technologies). NIS Elements was used to quantify cell densities and measure cell length. Cell staining with cytoplasmic cyclin B1 or H3P was quantified per 10,000 cells using Nikon Elements Software.
FACScan
One million MDCK II cells were collected by trypsin treatment and rinsing twice with 2 ml PBS. The cell pellet was resuspended in 250 μl cold PBS and fixed for 2 h with 800 μl ice-cold 100% ethanol at −20 °C. The tubes were warmed to 37 °C for 10 min and cells were washed twice with 1 and 2 ml PBS. The cell pellet was resuspended in 500 μl propidium iodide (PI) staining solution (50 μg ml−1 PI, 0.1% Triton-X, 0.2 mg ml−1 RNase A in PBS), vortexed and incubated for 20 min at 37 °C. The samples were filtered and then analysed for propidium iodide fluorescence with a BD FACScan Analyzer.
Piezo1 knockdown and western blot analysis
Zebrafish (Danio rerio): For CRISPR-mediated knockdown of Piezo1, exon 2 of the zebrafish piezo1 gene was targeted for CRISPR knockdown and the target sequence was GTAGCGAAATATGCAGGctgagg (exonic sequence is uppercase, intronic sequence is lowercase). The 20 nucleotides minus the NGG motif were cloned into a plasmid containing the RNA loop structure required for recognition by the Cas9 enzyme and a T7-binding site, which allowed for the synthesis of single-guide RNA (sgRNA). To induce knockdown of Piezo1, 75 pg of sgRNA and 1,200 pg of Cas9-NLS encoding RNA were injected at the 1-cell-stage into wild-type (AB) embryos and mitosis rates were measured in mosaic F0 larvae at day 4 after fertilization. For Cas9-NLS mRNA synthesis, pT3Ts-nCas9n was obtained from the Chen laboratory via Addgene (46757).
Piezo1 morphants were made by mixing 4 ng translation blocking morpholino 1:1 with a 25 bp antisense containing a photolinker and a 4 bp mismatch, and injecting into 1-cell-stage wild-type (AB) embryos. At 28–32 hours after fertilization, embryos were exposed to 350 nm light for 20 s to activate the morpholino, then fixed and immunostained at 4 days post-fertilization. All experiments on zebrafish were done with accordance to our zebrafish IACUC protocol, approved by the University of Utah IACUC board.
MDCKs: siRNA to canine Piezo1 mRNA (from Sigma to the sense sequence GUGCUAUGGUCUCUGGGAUdTdT) was transfected with RNAiMAX (Thermofisher) into MDCK cells 1 day after plating. For rescue, 1 μg GFP–Piezo1 construct (gift from A. Patapoutian) was transfected after 1 day after addition of siRNA. Alternatively, a pTRIPZ human PIEZO1 lentiviral plasmid (from Fisher Scientific to the sense sequence UGUCCUCCACACACAUCCA) was transduced with psPAX and p60 (gift from M. VanBrocklin) into MDCK cells and selected for 14 days with 2 μg ml−1 puromycin, and expression was induced by treating with with 1.5 μg ml−1 doxycycline (Sigma) for 3 days. Cell lysate was obtained by trypsinising cells, resuspending the pellet in NP40 cell lysis buffer containing 1× PMSF and protease inhibitors (Life Technologies), and centrifuging for 10 min at 16,873g at 4 °C. Then 30 μg of protein lysate (determined by Bradford assay) was run on a 3–8% Tris-acetate gel at 150 V for 1 h, transferred to PVDF, and probed for Piezo1 (Proteintech) or mouse-anti-tubulin (Sigma) antibody by standard enhanced chemiluminescence.
Statistical analysis
No statistical methods were used to predetermine sample size. For statistical analysis, all experiments, including those in zebrafish, were repeated on three separate days to ensure enough variation and numbers to measure significance by unpaired t-tests. To ensure randomization of measurements, we took random pictures within the middle (region of consistent stretch or crowding) of silicone membrane for quantification. For zebrafish experiments, all fish were analysed, so not randomized or blinded. After noticing a trend in low-density epithelia not responding to stretch, we excluded them in our main data, but included them in Supplementary Fig. 4. Variance between compared samples was similar and expressed as s.e.m. in graphs.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request; see author contributions for specific datasets. The source data for the western blots in the figures are available in Supplementary Fig. 1.
References
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012)
Puliafito, A. et al. Collective and single cell behavior in epithelial contact inhibition. Proc. Natl Acad. Sci. USA 109, 739–744 (2012)
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010)
Shah, A. N., Davey, C. F., Whitebirch, A. C., Miller, A. C. & Moens, C. B. Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 12, 535–540 (2015)
Shah, A. N., Moens, C. B. & Miller, A. C. Targeted candidate gene screens using CRISPR/Cas9 technology. Methods Cell Biol. 135, 89–106 (2016)
Benham-Pyle, B. W., Pruitt, B. L. & Nelson, W. J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348, 1024–1027 (2015)
Azzolin, L. et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014)
Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013)
Coste, B. et al. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 6, 7223 (2015)
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012)
Ge, J. et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527, 64–69 (2015)
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011)
Agell, N., Bachs, O., Rocamora, N. & Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell. Signal. 14, 649–654 (2002)
Chuderland, D., Marmor, G., Shainskaya, A. & Seger, R. Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J. Biol. Chem. 283, 11176–11188 (2008)
Chuderland, D. & Seger, R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun. Integr. Biol. 1, 4–5 (2008)
Shapiro, P. S. et al. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J. Cell Biol. 142, 1533–1545 (1998)
Wright, J. H. et al. Mitogen-activated protein kinase kinase activity is required for the G2/M transition of the cell cycle in mammalian fibroblasts. Proc. Natl Acad. Sci. USA 96, 11335–11340 (1999)
Hayne, C., Tzivion, G. & Luo, Z. Raf-1/MEK/MAPK pathway is necessary for the G2/M transition induced by nocodazole. J. Biol. Chem. 275, 31876–31882 (2000)
Hart, Y. & Alon, U. The utility of paradoxical components in biological circuits. Mol. Cell 49, 213–221 (2013)
Hart, Y., Antebi, Y. E., Mayo, A. E., Friedman, N. & Alon, U. Design principles of cell circuits with paradoxical components. Proc. Natl Acad. Sci. USA 109, 8346–8351 (2012)
Hart, Y. et al. Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell 158, 1022–1032 (2014)
Cheng, Y. et al. Analysis of DNA methylation patterns associated with the gastric cancer genome. Oncol. Lett. 7, 1021–1026 (2014)
Abend, M. et al. Iodine-131 dose dependent gene expression in thyroid cancers and corresponding normal tissues following the Chernobyl accident. PLoS One 7, e39103 (2012)
Donnard, E. et al. Mutational analysis of genes coding for cell surface proteins in colorectal cancer cell lines reveal novel altered pathways, druggable mutations and mutated epitopes for targeted therapy. Oncotarget 5, 9199–9213 (2014)
Spier, I. et al. Exome sequencing identifies potential novel candidate genes in patients with unexplained colorectal adenomatous polyposis. Fam. Cancer 15, 281–288 (2016)
Pathak, M. M. et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl Acad. Sci. USA 111, 16148–16153 (2014)
Held, M. et al. CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nat. Methods 7, 747–754 (2010)
Acknowledgements
We thank D. Wright for design consultation and printing our new stretch device prototypes, M. Yoshigi for the previous stretch device, L. Zinn-Bjorkman for video analysis, A. Patapoutian for a GFP–Piezo1 construct, D. Morgan and B. Edgar for cell cycle experimental advice and B. Edgar for comments on the manuscript. A National Institute of Health Director’s New Innovator Award 1DP2OD002056-01, R01GM102169, and University of Utah Funding Incentive Seed Grant to J.R. and P30 CA042014 awarded to Huntsman Cancer Institute core facilities supported this work. We thank the Fluorescence Microscopy and Mutation Generation and Detection Cores in the Health Sciences Cores at the University of Utah. An NCRR Shared Equipment Grant 1S10RR024761-01 paid for microscopy equipment.
Author information
Authors and Affiliations
Contributions
J.R. designed all of the experiments, interpreted, and analysed all data, and wrote the manuscript. J.R. and J.L. designed the new stretch device. J.L. and S.A.G. performed most stretch and wound healing experiments and FACS analysis throughout the paper. P.D.L. performed the first experiments showing stretch induces cell division. K.E. performed the immunoblots and the EdU experiments. V.K. did calcium imaging. C.F.D. produced F0 CRISPR mosaic knockout zebrafish, M.J.R. injected morpholinos into zebrafish embryos and helped with live imaging. J.R. performed the experiments using zebrafish and requirement for Piezo1 at steady state and co-quantified most of the experiments. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Profile of epithelial cell proliferation and cell density over time.
a, Daily proliferation rates of MDCK cells, as measured by H3P immunostaining, in which the asterisk indicates when confluence is reached (n = 3). Cell division slows but does not stop at day 5. b, Cell density plateaus by 5 days of growth when cells reach around 11 per 1,000 μm2 (n = 3). All values are means of 10 fields of view from 3 independent experiments; error bars denote s.e.m. c, Cell cycle profiles by FACS at days 1 and 5 after plating at high density.
Extended Data Figure 2 Epithelial cells divide in sparsest regions of epithelia.
a–c, Density of cells in dividing versus non-dividing regions of epithelia, as measured by the cell length along the longest axis of the cell in four videos (except fixed colon sections) from human colon tissue (n = 8; a), developing zebrafish epidermis (n = 50; b), and MDCK cells in culture (n = 50; c). ****P < 0.0001, unpaired t-test. Error bars denote s.e.m.
Extended Data Figure 3 Stretching steady-state monolayers.
a, Left, new printable device used for stretching cells uniaxially on flexcell plates, unassembled (top) and assembled in the stretched state (bottom). Right, schematic of uniaxial stretch on an epithelium in the unassembled (top) and assembled (bottom) states (photo credit: J.R.). b, Immunostained MDCK monolayers before (top) and after (bottom) stretching. Images are representative of more than 200 captured images each. Scale bar, 10 μm.
Extended Data Figure 4 Piezo1 morphants have reduced cell division in zebrafish epidermis at the steady state.
a, Cell division in zebrafish reaches a low steady state at days 4–5 after fertilization. n = 50 fish each day; error bars denote s.e.m. b, Photo-activation of zebrafish injected with a Piezo1 translation-blocking morpholino (MO) results in knockdown of Piezo1 protein, as shown by an immunoblot. See Supplementary Fig. 1 for full blots. c, d, Zebrafish Piezo1 morphants have notably reduced epidermal mitoses at 5 days post-fertilization when cells homeostatic growth rate. Values in c are means from 3 separate experiments, error bars denote s.e.m. ****P < 0.005, unpaired t-test. Each micrograph in d is representative of approximately 75 samples. Scale bars, 100 μm.
Extended Data Figure 5 A single calcium spark occurs around 1 h before cells divide.
a, Blocking stretch-induced proliferation with gadolinium (Gd3+) at 2 h after stretch does not affect the percentages of cells in the S phase, as measured by EdU incorporation. Data are mean and s.e.m. from 6 independent experiments. P > 0.05, t-test (compared to non-stretched control). b, Knockdown (KD) of Piezo1 with Gd3+ or inhibition of transcription with α-amanitin blocks stretch-induced cytoplasmic cyclin B accumulation and mitosis (H3P). Micrographs are representative of more than 100 samples, except for α-amanitin (40 samples). Scale bar, 10 μm. c, Quantification of time from calcium spark to cell rounding, measured from 10 mitotic events in 8 videos. Error bars denote s.e.m. d, Sample graph measuring the time (min) from calcium spark (arrow) to cell rounding (arrowhead) in MDCK cells expressing the calcium indicator CMV-R-GECO1 using Nikon Elements Software.
Extended Data Figure 6 Models for how Piezo1 controls cell division in response to stretch, and cell extrusion in response to crowding.
a, Theoretical graph of density-dependent Piezo1 function for cell division and cell death. Epithelia trend to a steady-state density, X. If density is reduced, stretching causes Piezo1 to activate cell division; if density increases, crowding causes Piezo1 to activate cells to extrude and die. b, Schematic showing how Piezo1 (green) localizes to the plasma membrane in sub-regions of the epithelia in which cells are sparser, and divides and accumulates into cytoplasmic aggregates in sub-regions in which cells are older, crowded, and prone to extrude.
Extended Data Figure 7 Stretch causes cells at the steady state to enter mitosis.
a, Characterization of the Piezo1 antibody using a Piezo1 shRNA construct tagged with mCherry indicates that cells expressing the mCherry shRNA construct (red) lack Piezo1 (green). Micrographs are representative of approximately 50 samples. Scale bar, 10 μm. b, High-density MDCK monolayers (more than 500 cells per 40× field) in which the intrinsic proliferation rate is low (less than 50 H3P-positive cells per 10,000 cells) proliferate in response to mechanical stretch (left) or wounding (right). Error bars denote s.e.m. ***P = 0.0007, unpaired t-test. c, Low-density MDCK monolayers just reaching confluence with a higher intrinsic rate of proliferation (more than 50 H3P-positive cells per 10,000 cells) do not significantly proliferate in response to stretch or wounding. P > 0.05, t-test. n = 12 experiments for each case.
Supplementary information
Supplementary Information
This file contains the uncropped scans with size marker indications. (PDF 838 kb)
Cells of MDCK monolayer dividing just after reaching confluence
Phase time-lapse video of MDCK monolayer growing for three days that have just reached confluence but are still sparse and rapidly dividing. Frames are taken every 10 minutes for 18 hours. (MOV 6277 kb)
Cells of MDCK monolayer divide more slowly once they become denser
Phase time-lapse video of MDCK monolayer grown for four days (two days after reaching confluence) divide less frequently when more densely populated. Note cells are dividing in sparsest regions and extruding in most crowded regions. Frames are taken every 10 minutes for 18 hours. (MOV 7290 kb)
Cells dividing within zebrafish larval epidermis
Time-lapse video from a spinning disc confocal microscope of a KRT4:GFP zebrafish larva that expresses GFP in the epidermis. Cell division occurs in sparsest regions. One sample used for quantification in Extended Data Figure 2. (MOV 4040 kb)
Wound closure and cell divisions in a wild type MDCK monolayer
Phase time-lapse video of an MDCK monolayer grown for four days and scratched with a pipet tip. Notice that most of the cell divisions take place after the wound has completely closed. One of ten videos quantified in Figure 1 A. Frames are taken every 10 minutes for 18 hours. (MOV 5312 kb)
Wound closure and cell divisions in a Piezo1 knockdown MDCK monolayer
Phase time-lapse video of an MDCK monolayer grown for four days and scratched with a pipet tip. Notice that few cell divisions occur and cells continue to migrate after wound closure. One of 10 videos quantified in Figure 2. Frames are taken every 10 minutes for 24 hours. (MOV 2391 kb)
Wound closure and cell divisions in a wild type MDCK monolayer
Phase time-lapse video of an MDCK monolayer grown for four days and scratched with a pipet tip. Note that most of the cells divide after the wound has completely closed and start to extrude when they become crowded later. Frames are taken every 10 minutes for 24 hours. (MOV 3425 kb)
Rights and permissions
About this article
Cite this article
Gudipaty, S., Lindblom, J., Loftus, P. et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121 (2017). https://doi.org/10.1038/nature21407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21407
This article is cited by
-
“Patchiness” in mechanical stiffness across a tumor as an early-stage marker for malignancy
BMC Ecology and Evolution (2024)
-
Cellular and molecular mechanisms of skin wound healing
Nature Reviews Molecular Cell Biology (2024)
-
PIEZO1 loss-of-function compound heterozygous mutations in the rare congenital human disorder Prune Belly Syndrome
Nature Communications (2024)
-
Homeostatic regulation of renewing tissue cell populations via crowding control: stability, robustness and quasi-dedifferentiation
Journal of Mathematical Biology (2024)
-
Piezo1 expression in chondrocytes controls endochondral ossification and osteoarthritis development
Bone Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.